
Legend (Hyaluronate Sodium) Injectable Solution, 4 ml
 Limited Time Sale
Limited Time Sale$74.79 cheaper than the new price!!
Free cash-on-delivery fees for purchases over $99
Product details
| Management number | 204570229 | Release Date | 2025/10/14 | List Price | $61.20 | Model Number | 204570229 | ||
|---|---|---|---|---|---|---|---|---|---|
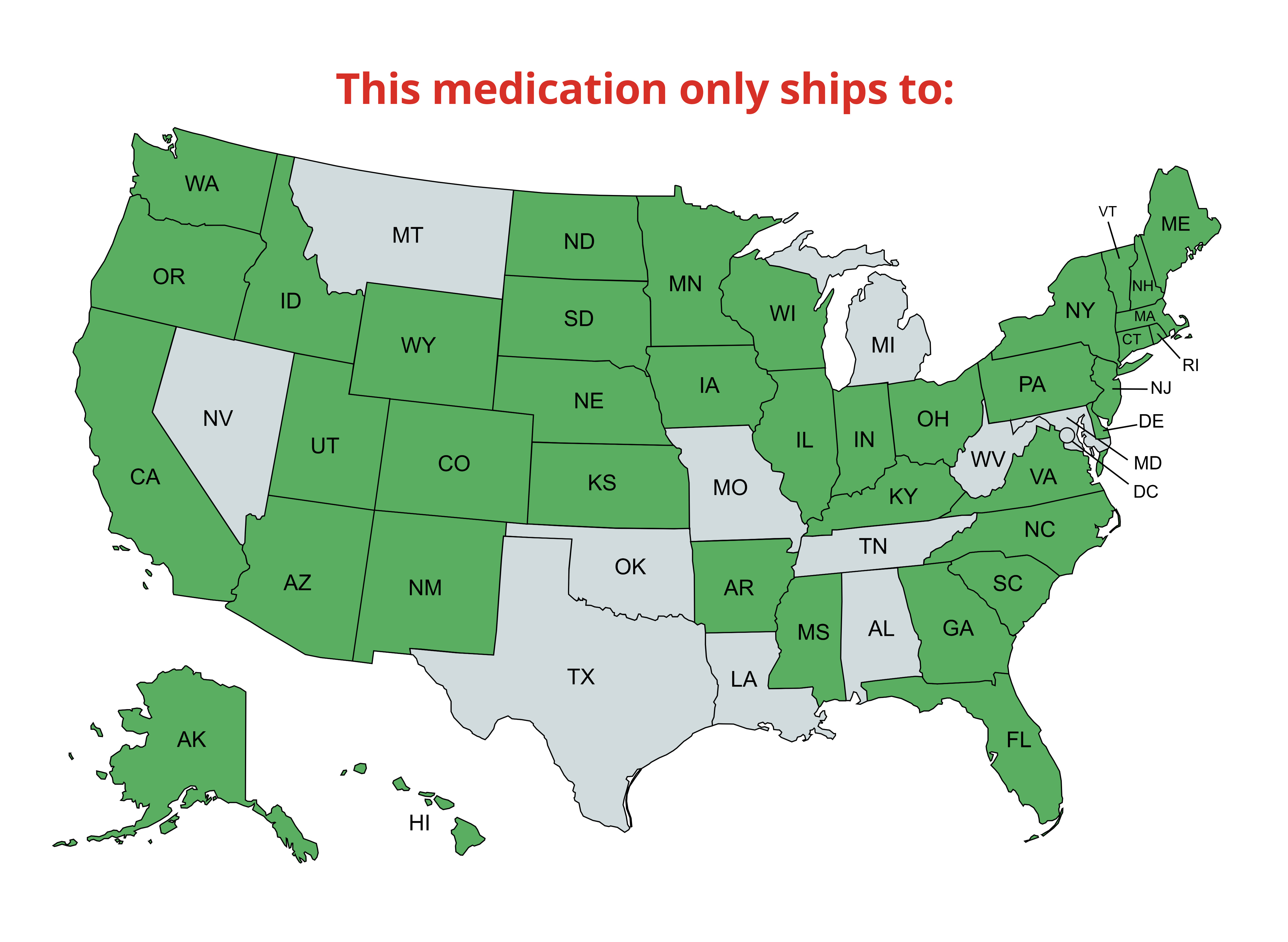
| Category | |||||||||
Legend (Hyaluronate Sodium) Injectable Solution, 4 ml
LEGEND Injectable Solution is indicated in the treatment of joint dysfunction of the carpus or fetlock in horses due to non-infectious synovitis associated with equine osteoarthritis. Equine osteoarthritis can happen in any age and any discipline due to everyday wear and tear. It is permanent and can lead to early retirement and quality-of-life issues in horses.
The active ingredient in LEGEND, hyaluronic acid (HA), decreases the presence of inflammatory mediators in the joint, improves synovial fluid quality, and reduces the degree of lameness. These improvements are seen whether LEGEND is administered by intravenous (I.V.) or intra-articular (I.A.) injection. LEGEND hyaluronate sodium injectable is sterile, pyrogen-free and FDA-approved.
Benefits
-
Safe and efficacious
-
90% of cases treated I.V. judged to have excellent to good overall clinical improvement1
-
96% of horses treated intra-articularly were judged to have excellent to good overall clinical improvement1
-
Improvement can be seen after one dose
-
Provides multi-modal approach against the cycle of inflammation and lameness
-
Stimulates endogenous hyaluronic acid production2
-
Creates a physical barrier to exclude inflammatory mediators3
-
Decreases the presence of inflammatory mediators4
-
Easy to use I.V. administration for when intra-articular administration is not practical or desired
Recommended Dosage
Use as directed by your veterinarian.
LEGEND 4 mL (40 mg) can be injected intravenously only at a dosage of 4 mL. Intravenous treatment may be repeated at weekly intervals for a total of 3 treatments.
Correction of product information
If you notice any omissions or errors in the product information on this page, please use the correction request form below.
Correction Request Form


















